If you’re like the rest of the team here at the National Wildlife Council, then you think bioluminescence is one of the coolest things ever.
That’s why we want to help you learn a bit more about this cool phenomenon that you see in nature.
There are all sorts of animals and plant life that are bioluminescent, and it helps to better understand what you’re seeing when you stumble upon it in the wild.
Bioluminescence vs Biofluorescence
Bioluminescence, or living light, is produced by a chemical reaction churning in the bodies of thousands of different organisms from terrestrial and marine environments around the planet.
This remarkable “cold” reaction is highly efficient, so despite radiant results, it generates almost no heat – nearly 100% of the energy consumed in the reaction is given off as the signature “cold light” of glowing organisms.
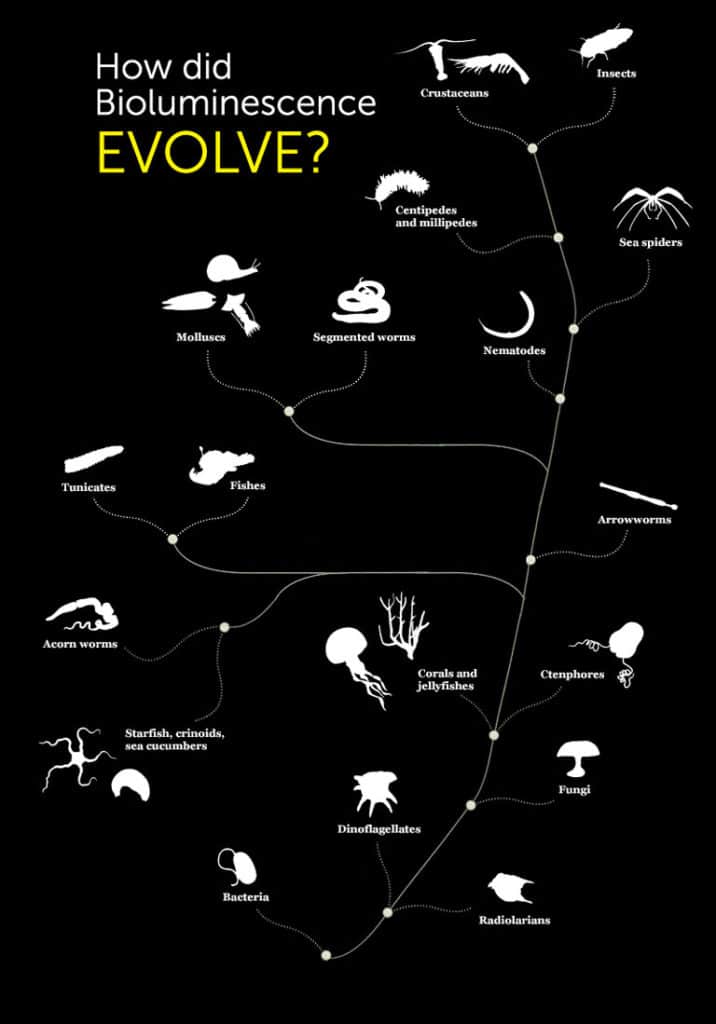
The illuminating phenomenon of bioluminescence is scattered across the tree of life.
Biofluorescence, on the other hand, is not a chemical reaction and biofluorescent organisms do not give off light from their own power source.
Instead, biofluorescent organisms absorb light, transform it, and eject – or “re-emit” – it as a different color.
When specialized fluorescent molecules are “excited” by high-energy light (like blue light), they lose a fragment of the light energy and release the rest at a lower-energy wavelength (like green).

The Science of Bioluminescence
At the molecular level, bioluminescence is produced when chemicals, or “substrates” intermingle at the right time and place: luciferin, luciferase, and a third player, oxygen.

Some organisms generate their own light through a photoprotein, which emits light in an oxygen- independent reaction already has the oxygen built in the photoprotein molecule.

The Science of Biofluorescence
Biofluorescent light can only be produced – and seen by humans – while the organism is being illuminated by an external source, such as a white or ultraviolet (UV) light bulb.

On the atomic level, an organism’s fluorescent molecules absorb the photons – tiny parcels of light – given off by the external source.
When they collide with the fluorescent molecule, the photons “excite” the molecule by bumping its electrons up to a higher energy state.

Once excited, these electrons quickly relax to their ground state, but as they relax, they release the extra energy as new photons or parcels of light.
Because they consumed energy during their “excitations,” the electrons re-emit photons at a lower energy level, or a longer wavelength of light.
Humans cannot see this excitation- relaxation process, which occurs almost instantaneously – between a trillionth and a millionth of a second.
But humans can see the photons’ changed energy state, which will appear as a different color of the visible light spectrum, or rainbow, than the color of the external light source.
Since light fuels fluorescence, fluorescent pigments only glow when they are under high energy radiation — like ultraviolet light.


